VSEPR Theory
The valence-shell electron-pair repulsion (VSEPR) model predicts the shapes of molecules and ions by assuming that the valence-shell electron pairs are arranged about each atom so that electron pairs are kept as far away from one another as possible, thus minimizing electron-pair repulsions.
The possible arrangements assumed by different numbers of electron pairs about an atom are shown below.
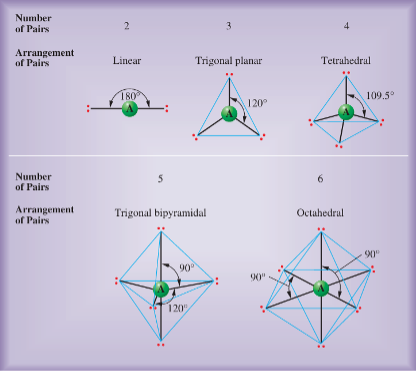
For example, if there are only two electron pairs in the valence shell of an atom, these pairs tend to be at opposite sides of the nucleus so that repulsion is minimized. This gives a linear arrangement of electron pairs; that is, the electron pairs mainly occupy regions of space at an angle of 180° to one another.
If three electron pairs are in the valence shell of an atom, they tend to be arranged in a plane directed toward the corners of a triangle of equal sides (equilateral triangle). This arrangement is trigonal planar, in which the regions of space occupied by electron pairs are directed at 120° angles to one another.
Four electron pairs in the valence shell of an atom tend to have a tetrahedral arrangement. That is, if you imagine the atom at the center of a regular tetrahedron, each region of space in which an electron pair mainly lies extends toward a corner, or vertex. (A regular tetrahedron is a geometrical shape with four faces, each an equilateral triangle.) Thus, it has the form of a triangular pyramid.) The regions of space mainly occupied by electron pairs are directed at approximately 109.5° angles to one another.
Molecular geometries
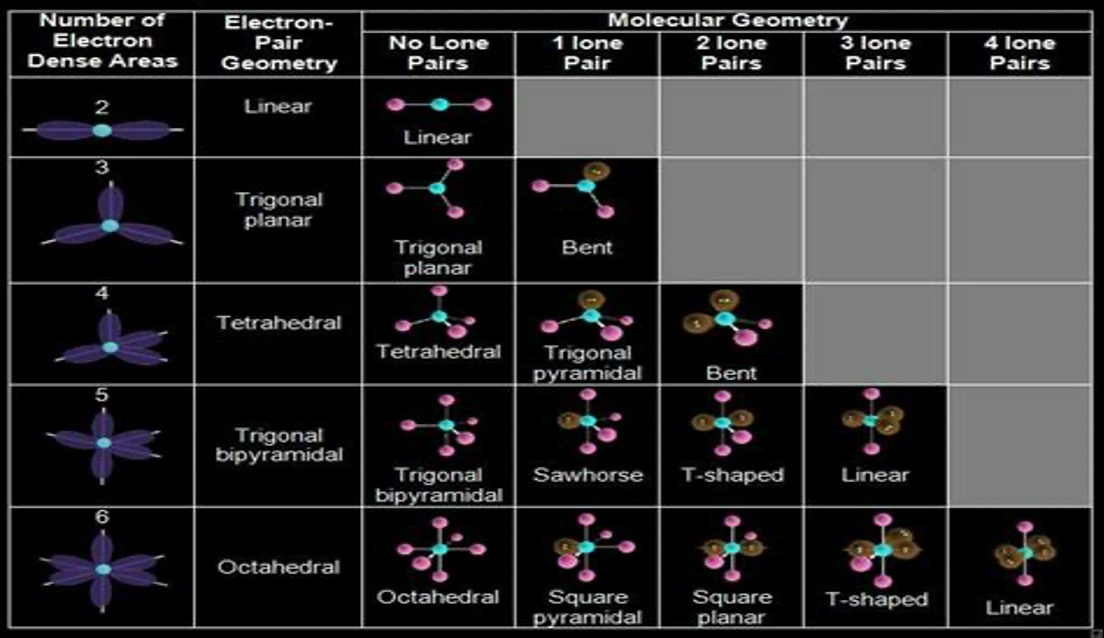
VSEPR theory and polarity
| Formula | Molecular Geometry | Dipole Moment |
|---|---|---|
| \(\ce{AX}\) | Linear | Can be nonzero |
| \(\ce{AX2}\) | Linear | Zero |
| Bent | Can be nonzero | |
| \(\ce{AX3}\) | Trigonal planar | Zero |
| Trigonal pyramidal | Can be nonzero | |
| T-shaped | Can be nonzero | |
| \(\ce{AX4}\) | Tetrahedral | Zero |
| Square planar | Zero | |
| Seesaw | Can be nonzero | |
| \(\ce{AX5}\) | Trigonal bipyramidal | Zero |
| Square pyramidal | Can be nonzero | |
| \(\ce{AX6}\) | Octahedral | Zero |
(For example, linear, trigonal planar, and tetrahedral) give molecules of zero dipole moment; that is, the molecules are nonpolar. Those geometries in which the X atoms tend to be on one side of the molecule (for example, bent and trigonal pyramidal) can have nonzero dipole moments; that is, they can give polar molecules.
Written by Fillios Memtsoudis